Selected publications
Below are some selected highlights from our publications. A full publication list can be found on Google Scholar or ORCID.
Below are some selected highlights from our publications. A full publication list can be found on Google Scholar or ORCID.
Twelvetrees AE
Seminars in Cell and Developmental Biology, (2020) doi.org/10.1016/j.semcdb.2020.02.008
This review provides an overview of the lifecycle of cytoskeletal components in neurons, focusing on its spatial organisation over time in the axon.
Twelvetrees AE, Lesept F, Holzbaur ELF and Kittler JT
Journal of Cell Science, 132: jcs215822 (2019) doi.org/10.1242/jcs.215822
The motor protein kinesin-1 frequently uses cytosolic proteins called adaptor proteins to attach to organelles such as vesicles. However although many potential adaptors have been identified because of their ability to bind to kinesin, very few have been shown to unfold and activate the motor. Two adaptor proteins critical for neuronal function are glutamate receptor interacting protein 1 (GRIP1) and huntingtin-associated protein 1 (HAP1). GRIP1 links excitatory AMPARs to kinesin-1 for delivery to excitatory synapses (Setou et al., 2002), whereas HAP1 is an adaptor between inhibitory GABAARs and kinesin-1 (Twelvetrees et al., 2010). In this paper we showed that GRIP1 and HAP1 form an endogenous kinesin-activating complex by binding distinct sites on the kinesin heavy chain. Using in vitro studies, we found that HAP1 and GRIP1 work together to activate kinesin.
Sleigh JN, Vagnoni A, Twelvetrees AE, and Schiavo G
F1000Research, 6: 200 (2017) doi.org/10.12688/f1000research.10433.1
This review highlights recent advances in intravital imaging of axonal transport, outlining key strengths and limitations while discussing findings, possible improvements, and outstanding questions.
Twelvetrees AE, Pernigo S, Sanger A, Guedes-Dias P, Schiavo G, Steiner RA, Dodding MP and Holzbaur ELF
Neuron, 90(5):1000-15 (2016) dx.doi.org/10.1016/j.neuron.2016.04.046
See also the preview article by Subhojit Roy, Neuron 90(5):907-909 (2016) doi.org/10.1016/j.neuron.2016.05.026
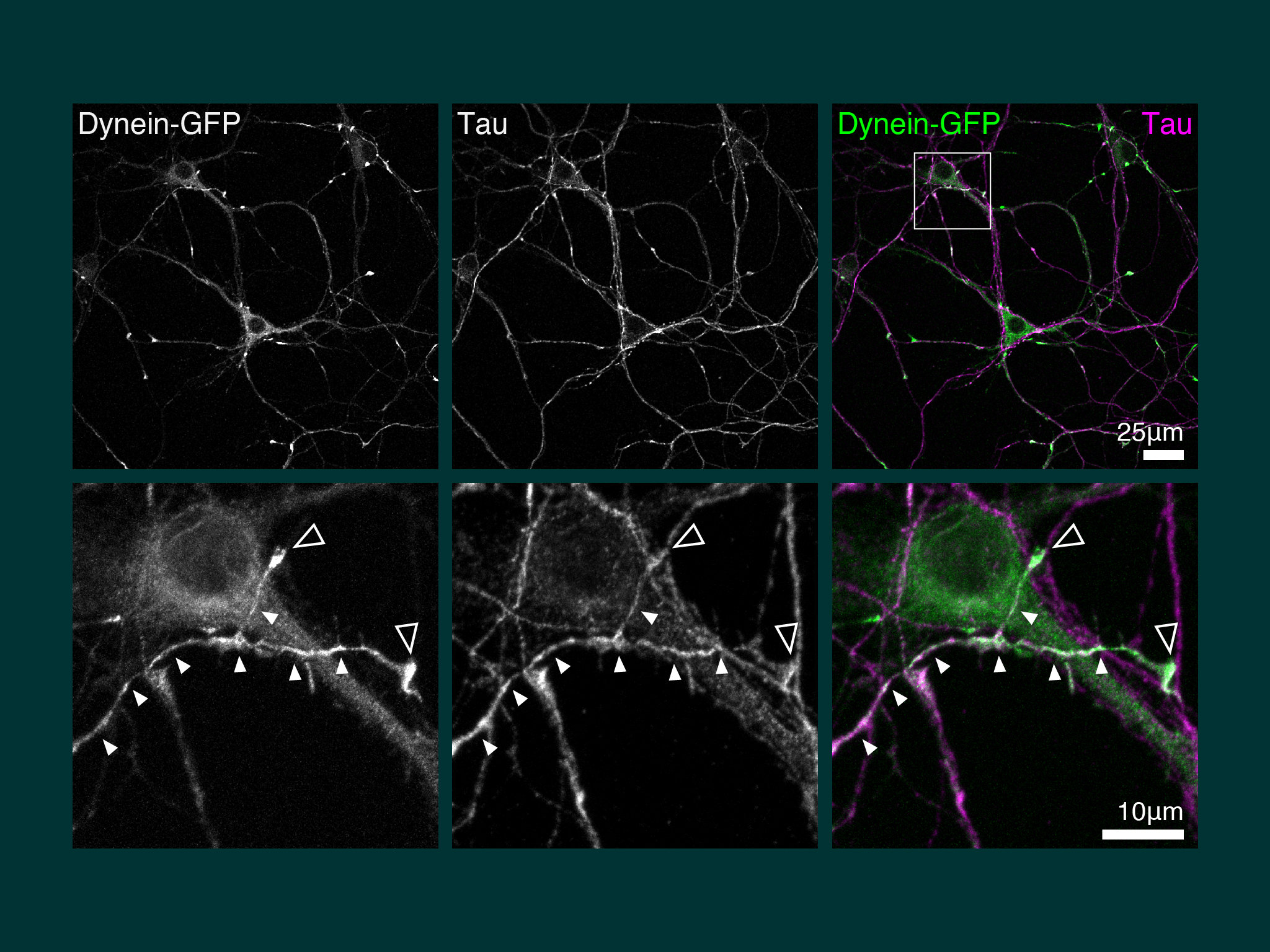
Our first discovery when we cultured hippocampal neurons from the dynein-GFP mouse, was pronounced accumulation of dynein in the the distal axon.
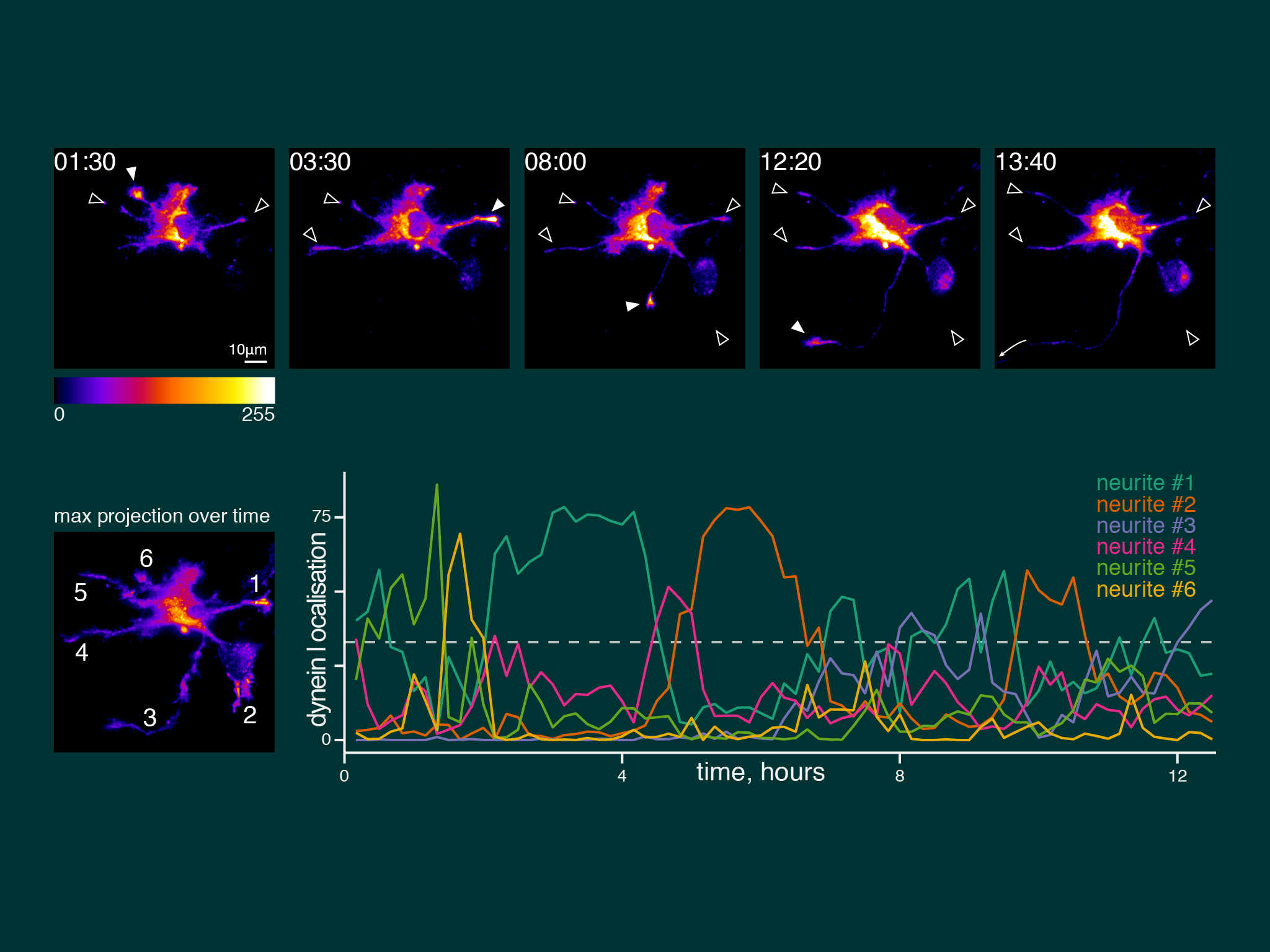
When we imaged neuronal development over many hours, dynein accumulation was dynamic between neurites. However, dynein was present coincident with axon speification.
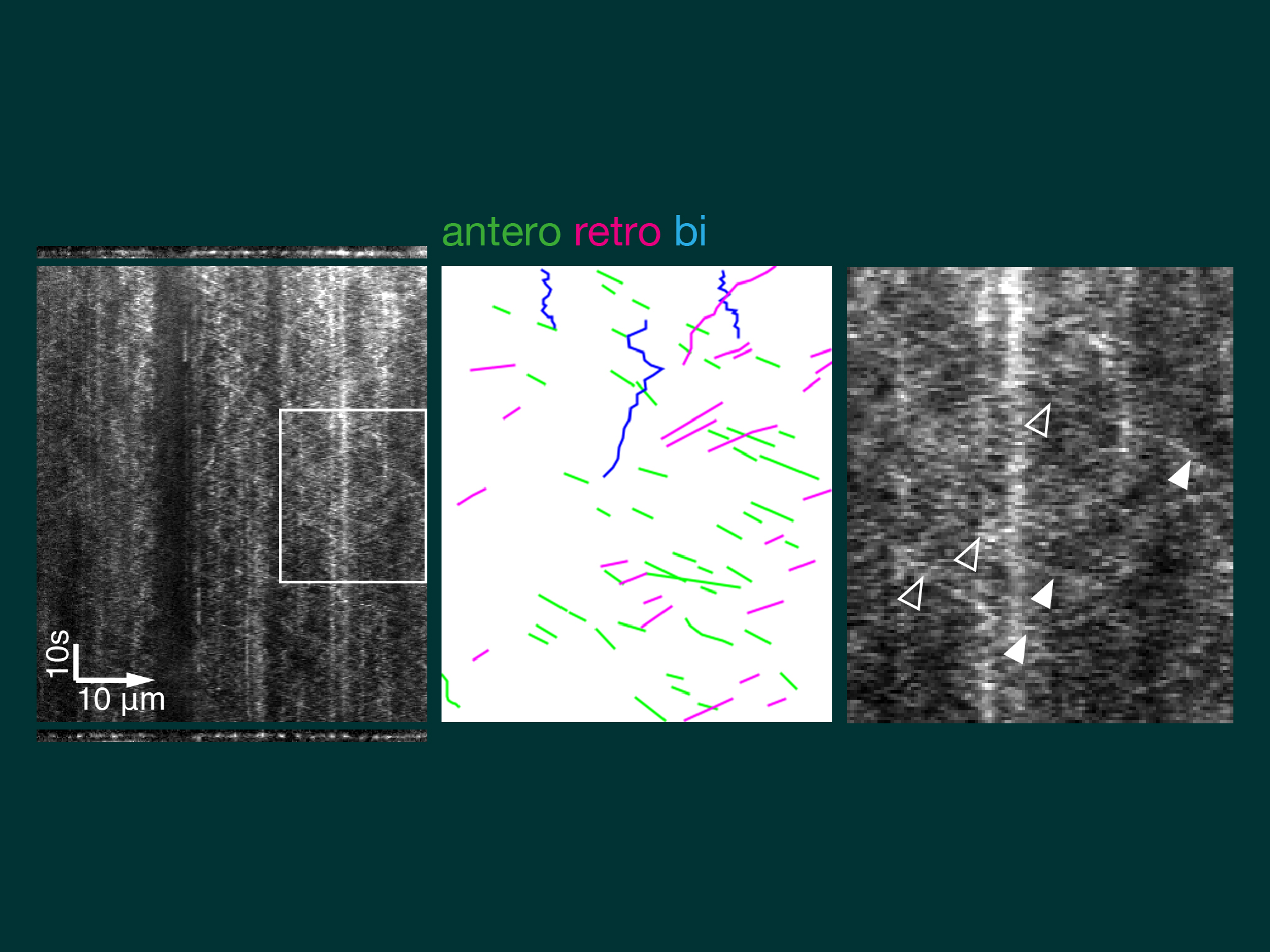
Kymographs of axons imaged by near TIRF (HiLo) show dynein moving by short anterograde events of comparatively uniform velocity and low signal intensity. Typical speed was ~1 um/s, equivalent to the known speed of kinesin-1.
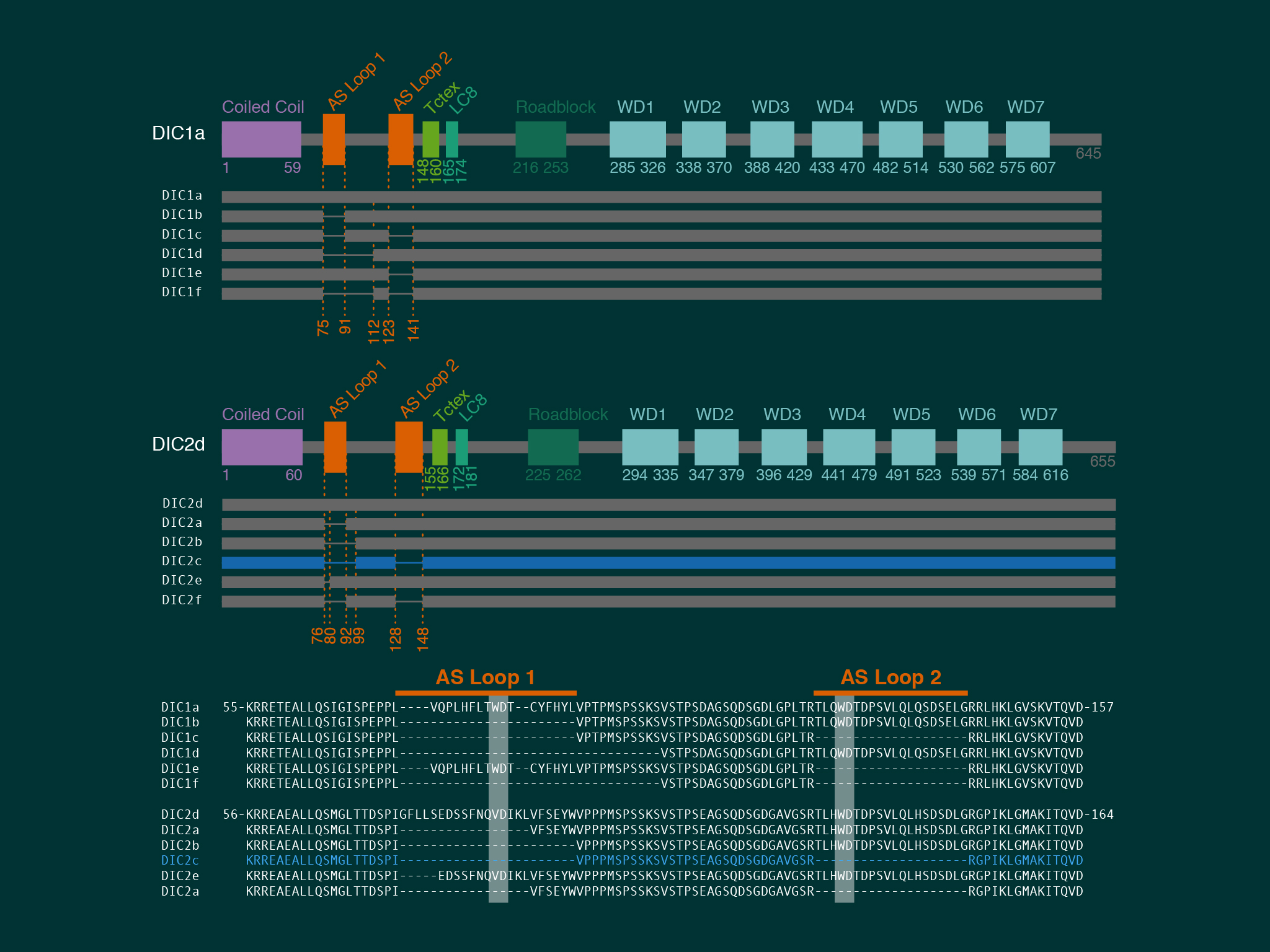
Using extensive biochemistry we found that the direct interaction between dynein and kinesin was mediated by neuron specific isoforms of the dynein intermediate chain.
Neurons treated with a peptide blocking the interaction between kinesin and dynein showed a large reduction in dynein anterograde trasnport speeds.
We describe a model of slow axonal transport whereby kinesin is essentially in rate limiting supply for slow transport cargoes, producing short bursts of motility and low cargo net transport speeds.
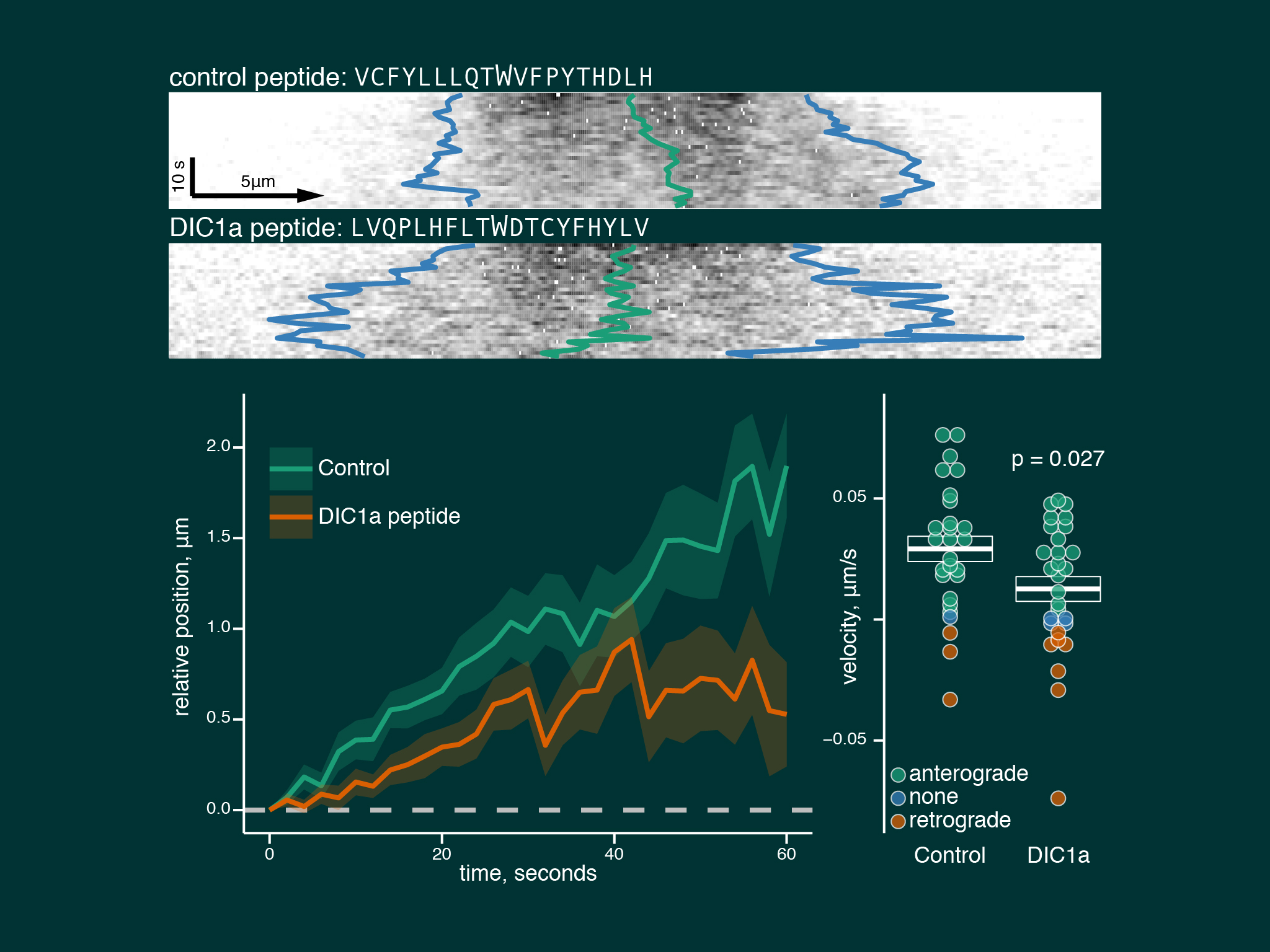
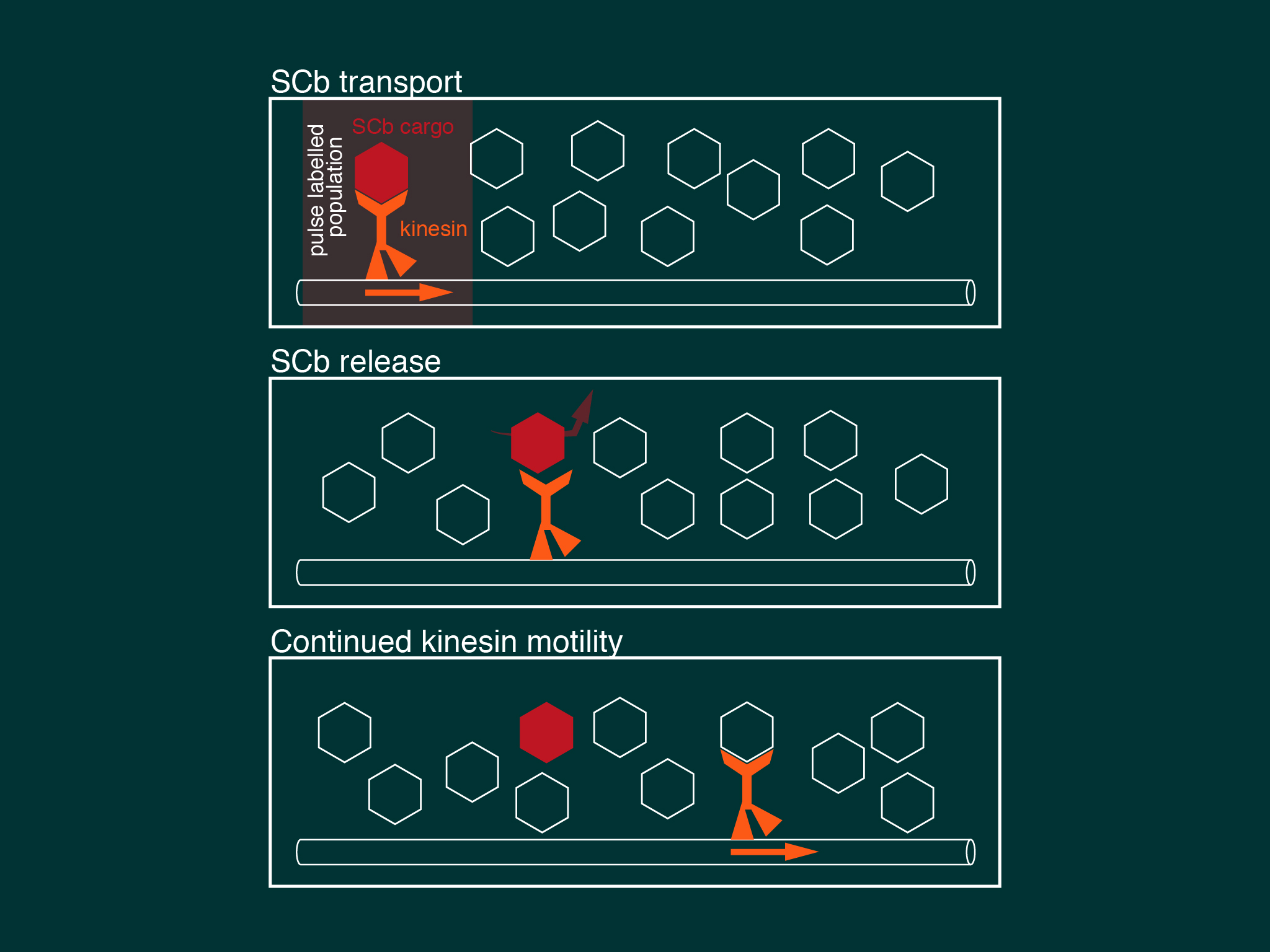
⬇︎ You can also watch a summary of our findings in our featured video abstract.
The highly organised microtubule polarity of the axon means that the microtubule motors dynein and kinesin have a defined stepping direction when they hydrolyse ATP; kinesin moves away from the cell body whilst dynein can only move back. Cytoplasmic dynein is the major retrograde motor in axons, however as it is synthesised in the cell body it cannot drive its own localization to the axon terminal. This is a fundamental problem that neurons have to overcome because dynein is essential for many axonal functions including: growth cone extension (Grabham et al., 2007; Myers et al., 2006); axon elongation (Roossien et al., 2014); retrograde neurotrophic signaling (Heerssen et al., 2004; Yano et al., 2001); and autophagy (Maday et al., 2012). Our paper described the molecular mechanism of how dynein is localized to the distal axon.
For this work we took advantage of the dynein-GFP mouse (see below). This knock-in mouse model incorporates a GFP tag to label an endogenous neuron specific subunit of dynein, allowing direct observation of dynein expressed at endogenous levels for the first time. Using primary hippocampal neurons as a model system, dynein was imaged over a broad range of spatio-temporal scales: i) throughout neuronal development over many hours; ii) during axonal transport in real time; iii) as single molecules in vitro. When coupled to novel quantitative methods, this powerful strategy allowed the discovery of how the dynamic localization of neuronal dynein is driven by direct interactions with kinesin-1, mediated by neuron specific isoforms of the dynein intermediate chain.
Maday S, Twelvetrees AE, Moughamian AJ and Holzbaur ELF
Neuron, 84(2):292-309 (2014) dx.doi.org/10.1016/j.neuron.2014.10.019

Axonal transport is essential for neuronal function, and many neurodevelopmental and neurodegenerative diseases result from mutations in the axonal transport machinery. Anterograde transport supplies distal axons with newly synthesized proteins and lipids, including synaptic components required to maintain presynaptic activity. Retrograde transport is required to maintain homeostasis by removing aging proteins and organelles from the distal axon for degradation and recycling of components. Retrograde axonal transport also plays a major role in neurotrophic and injury response signaling. This review provides an overview of axonal transport pathways and discusses their role in neuronal function.
Zhang J, Twelvetrees AE, Lazarus JE, Blasier KR, Yao X, Inamdar NA, Holzbaur ELF, Pfister K and Xiang X
Cytoskeleton (Hoboken, NJ) 70, 215–227 (2013) dx.doi.org/10.1002/cm.21102

This paper describes the establishment of the dynein-GFP mouse; a novel knock-in mouse line, where the neuron-specific cytoplasmic dynein 1 intermediate chain 1 (DIC1) is tagged with both GFP and a 3xFLAG tag at its C-terminus. As a knock-in model the fusion gene is under the control of DIC1s endogenous promoter and is integrated at the endogenous locus of the DIC1 gene, Dync1i1. Work in this paper showed that the DIC1-GFP-3xFLAG fusion protein is incorporated successfully into the endogenous dynein complex, allowing real time observation of dynein expressed at endogenous levels for the first time.
Twelvetrees AE, Hendricks AG and Holzbaur ELF
Cell 149, 950–950.e951 (2012) dx.doi.org/10.1016/j.cell.2012.05.001
In this ‘Snapshot’ review of axonal transport, we summarise visually some of the key properties of axonal transport: which cargoes use which motors; the key biophysical parameters describing those motors; and how long it takes different classes of cargo to reach the presynapse in different axons. Click to enlarge the image or download the pdf.
Hendricks AG, Twelvetrees AE and Holzbaur ELF
Current Biology 22, R1053–R1055 (2012) dx.doi.org/10.1016/j.cub.2012.11.009
Twelvetrees AE, Yuen EY, Arancibia-Carcamo IL, Macaskill AF, Rostaing PP, Lumb MJ, Humbert SS, Triller AA, Saudou F, Yan ZZ and Kittler JT
Neuron 65, 53–65 (2010) dx.doi.org/10.1016/j.neuron.2009.12.007

GABA-A receptors are the main sites of fast synaptic inhibition in the brain and so are critical for maintaining a neuron's excitatory/inhibitory balance. This paper identified kinesin-1 dependent transport of GABA-A receptors to synapses as a key mechanism controlling the numbers of GABA-A receptors at synapses, and the strength of synaptic inhibition. We discovered that GABA-A receptors associate with kinesin-1 via the specific adaptor protein Huntingtin Associated Protein 1 (HAP1). HAP1 is also known as an interaction partner for huntingtin, which causes Huntington’s disease through a polyglutamine (polyQ) expansion in the huntingtin protein. We found that in neurons expressing polyQ-huntingtin there was reduced GABA-A receptor trafficking, resulting in reduced synaptic inhibition. This mechanism may contribute to the excitatory/inhibitory imbalance in HD which causes excitotoxicity and neuronal death.
Macaskill AF, Rinholm JE*, Twelvetrees AE*, Arancibia-Carcamo IL, Muir J, Fransson A, Aspenstrom P, Attwell D and Kittler JT
Neuron 61, 541–555 (2009) dx.doi.org/10.1016/j.neuron.2009.01.030
See also article recommendation on F1000 ($).
